Main group elements
The First 36 Main Group Elements: Hydrogen to Barium
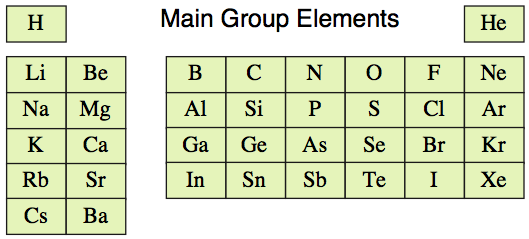
Initially we shall limit our
initial discussions to s and p-block elements, to first 36 main group
elements, hydrogen to barium:
However, as simple substances
in their standard state (1atm pressure and 25°C) the main group elements
present as a diverse and complicated collection of substances:
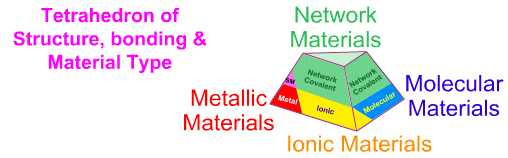
As discussed elsewhere in this
web book – see the pages on the Classification
of Matter and the Tetrahedron of Structure, Bonding & Material Type
pages – elemental and binary substances exhibit four extreme material types
[metallic, ionic, molecular & network], however the elements present
as just: metallic, molecular & network:
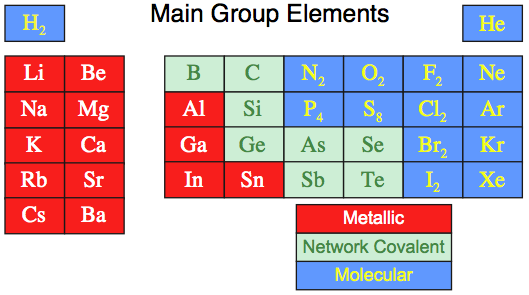
When this analysis is applied
to the set of the first 36 main group elements we find:
16 elements are molecular, 15 are metallic, 5 is network covalent (carbon) and none are ionic.
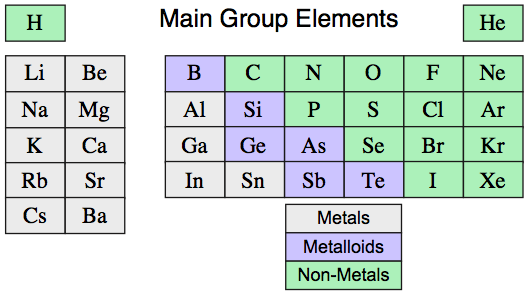
However, this is a gross simplification because several elements have metallic and non-metallic allotropes that are intermediate between metallic and network, and are metalloid or semi-metallic in nature: C, Si, Ge, As, Sn, Sb & Te.


Comments
Post a Comment