Main group element hydride
Q.1 Which of the following hydrides is electron-precise hydride ?
A.B₂H₆
B.NH₃
C.H₂O
D.CH₄
Answer:- D
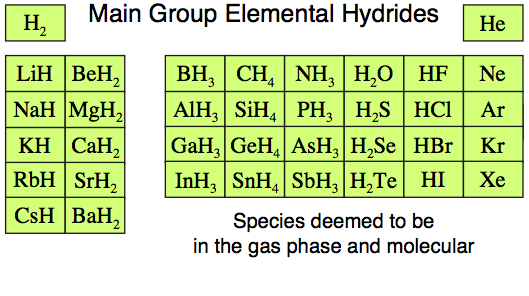
The Main Group Elemental Hydrides
The 36 of the main group elemental
hydrides, H2 to BaH2, are
all well known species with non-controversial structure and reaction chemistry.
Indeed, the set includes such common chemical species as water, H2O,
methane, CH4, ammonia, NH3,
hydrogen sulfide, H2S, hydrogen, H2,
and neon, Ne.
Of more importance – and
crucial to the normalisation argument – is that all the main group
elemental hydrides can be found in the gas phase where they are molecular.
This "gas phase
and molecular" point is crucial for two reasons:
- Most reaction chemistry involves molecules and/or molecular environments (the gas phase or in solution).
- Gas phase and molecular chemical species are simple to model, both on paper and in silico. This makes it possible to carry out computer aided virtual reaction chemistry.
Note:
- The Group
1 and 2 saline hydrides – lithium hydride to barium hydride, LiH
to BaH2 – are known to exist as ionic solids rather than as discrete
molecules. However, the saline hydrides and other ionic materials can
be studied in the gas phase molecular entities by laser
ablation of the crystal surface.
- Molecular lithium hydride, LiH,
is a very common species for theoretical study because it is the simplest
species with a heteronuclear bond, a bond between two dissimilar elements.
- The Group
13 hydrides such as borane, BH3, dimerise to B2H6.
- These seeming
exceptions to the molecular and in the gas phase argument are
actually core components of the chemogenesis argument, as should become
apparent.
- An additional
reason for restricting the current discussion to s and p-block elemental
hydrides, as discussed above, is that the d and f-block elements form
non-stoichiometric hydrides which can vary in composition. These interesting
materials are being studied for the storage of hydrogen.
The Main
Group Elemental Hydrides as: Lewis acids, Lewis bases and Lewis acid/base
Complexes
here.]
In the original
language of chemistry:
- A Lewis base is "a species with an electron pair".
- A Lewis acid is " an electron pair acceptor".
- A Lewis acid interact with a Lewis base to give a complex with a polar covalent, two electron chemical bond.
In the 1960s, frontier molecular
orbital (FMO) theory said:
- A Lewis base interacts with a Lewis acid via its highest occupied molecular orbital or HOMO.
- A Lewis acid interacts with a Lewis base via its lowest unoccupied MO or LUMO.
- HOMO + LUMO interactions are new bonding.
In the analysis presented here:
A Lewis acid's LUMO interacts with a Lewis base's HOMO to give a Lewis acid/base complex with a net bonding molecular orbital:
LUMO + HOMOLUMO/HOMO
bonding MO
There is a self-consistent
colour scheme running through chemogenesis:
- Lewis acids are RED
- Lewis bases are BLUE
- Lewis acid/base complexes are YELLOW
Hydride:
The term hydride is commonly named after binary compounds that hydrogen forms with other elements of the periodic table. Hydride compounds in general form with almost any element, except a few noble gases. The trends and properties vary according to the type of intermolecular force that bonds the elements together, the temperature, its molecular masses, and other components. Hydrides are classified into three major groups, depending on what elements the hydrogen bonds to. The three major groups are covalent, ionic, and metallic hydrides. Formally, hydride is known as the negative ion of a hydrogen, H-, also called a hydride ion. Because of this negative charge, hydrides have reducing, or basic properties. Its special characteristics will be further discussed.
Covalent Hydrides;
The first major group is covalent hydrides, which is when a hydrogen atom and one or more non-metals form compounds. This occurs when hydrogen covalently bonds to a more electropositive element by sharing electron pairs. These hydrides can be volatile or non-volatile. Volatile simply means being readily able to be vaporized at low temperatures. One such example of a covalent hydride is when hydrogen bonds with chlorine and forms hydrochloric acid (). Examples are listed below:
The hydrides of nonmetals on the periodic table become more electronegative as you move from group 13 to 17. This means that they are less capable of donating an electron, and want to keep them because their electron orbital becomes fuller. Instead of donating a , they would instead donate a because they are more acidic.
Example;Boron hydride
Boron can form many different types of hydrides; one of them is borane (), which reacts violently with air and is easily oxidized. Borane occurs as a gaseous substance, and can form by two borane molecules combined with each other. Borane is not a stable compound because it does not follow a complete octet rule since it has only six valence electrons.
Example 2;Nitrogen hydride
Ammonia is an important nitrogen hydride that is possible due to the synthesis of nitrogen and water which is called the Haber-Bosch process. The chemical equation for this reaction is:
To yield ammonia, there needs to be a catalyst to speed up the reaction, a high temperature and a high pressure. Ammonia is a reagent used in many chemistry experiments and is used as fertilizer. Ammonia can react with sulfuric acid to produce ammonium sulfate, which is also an important fertilizer. In this reaction, ammonia acts as a base since it receives electrons while sulfuric acid gives off electrons.
Other hydrides of nitrogen include ammonium chloride, hydrazine and hydroxylamine. Ammonium chloride is widely used in dry-cell batteries and clean metals.
Ionic Hydrides;
The second category of hydrides are ionic hydrides (also known as saline hydrides or pseudohalides). These compounds form between hydrogen and the most active metals, especially with the alkali and alkaline-earth metals of group one and two elements. In this group, the hydrogen acts as the hydride ion (). They bond with more electropositive metal atoms. Ionic hydrides are usually binary compounds (i.e., only two elements in the compound) and are also insoluble in solutions.
with as any group 1 metal.
with as any group 2 metal.
Ionic hydrides combine vigorously with water to produce hydrogen gas.
Example;Alkali metal hydride
As ionic hydrides, alkali metal hydrides contain the hydride ion as well. They are all very reactive and readily react with various compounds. For example, when an alkali metal reacts with hydrogen gas under heat, an ionic hydride is produced. Alkali metal hydrides also react with water to produce hydrogen gas and a hydroxide salt:
Metallic Hydrides;
The third category of hydrides are metallic hydrides, also known as interstitial hydrides. Hydrogen bonds with transition metals. One interesting and unique characteristic of these hydrides are that they can be nonstoichiometric, meaning basically that the fraction of H atoms to the metals are not fixed. Nonstoichiometric compounds have a variable composition. The idea and basis for this is that with metal and hydrogen bonding there is a crystal lattice that H atoms can and may fill in between the lattice while some might, and is not a definite ordered filling. Thus it is not a fixed ratio of H atoms to the metals. Even so, metallic hydrides consist of more basic stoichiometric compounds as well.



Comments
Post a Comment