Crystal packing
Q.If 'a' stands
for the edge length of the cubic systems: simple cubic, body centred cubic
and face centred cubic, then the ratio of the radii of the spheres in these
systems will be respectively:
A.
B.
C.
D.
Answer:Option-A
Atomic packing factor
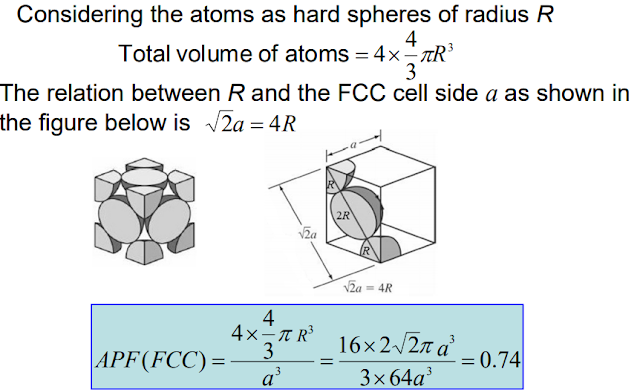
Atomic packing factor (APF) or packing efficiency indicates how closely atoms are packed in a unit cell and is given by the ratio of volume of atoms in the unit cell and volume of the unit cell
FCC lattice
In the FCC unit cell effective number of atoms = 8 corner atoms x (1/8) (each atom is shared by 8 unit cells) + 6 facecentered atoms x1/2 (each shared by two unit cells) = 4
Close-Packed Structure
FCC and hexagonal crystal structures are most highly packed with packing efficiency of 74% (APF= 0.74). Such structures can be described in terms of close-packed atomic planes.
In FCC, {111} planes are close-packed and the basal plane (0001) is the close-packed one in hexagonal close-packed (HCP) system. Therefore, both of these structures can be generated by stacking of these planes. A portion of such a stack is shown in the picture below.
Close-Packed Structure
There are two types of voids between the atoms – vertex up ( b), and vertex down ( c). The atoms in the next layer sit on the b sites (See animation below).
In FCC, atoms in the third layer sit over the c sites and this repeats giving rise to ABC ABC ABC type of stacking.
A.
B.
C.
D.
Answer:Option-A
Atomic packing factor
Atomic packing factor (APF) or packing efficiency indicates how closely atoms are packed in a unit cell and is given by the ratio of volume of atoms in the unit cell and volume of the unit cell
FCC lattice
In the FCC unit cell effective number of atoms = 8 corner atoms x (1/8) (each atom is shared by 8 unit cells) + 6 facecentered atoms x1/2 (each shared by two unit cells) = 4
Planar dencity
FCC and hexagonal crystal structures are most highly packed with packing efficiency of 74% (APF= 0.74). Such structures can be described in terms of close-packed atomic planes.
In FCC, {111} planes are close-packed and the basal plane (0001) is the close-packed one in hexagonal close-packed (HCP) system. Therefore, both of these structures can be generated by stacking of these planes. A portion of such a stack is shown in the picture below.
Close-Packed Structure
There are two types of voids between the atoms – vertex up ( b), and vertex down ( c). The atoms in the next layer sit on the b sites (See animation below).
In FCC, atoms in the third layer sit over the c sites and this repeats giving rise to ABC ABC ABC type of stacking.











Comments
Post a Comment