Oxygen transport and Storage -Hemoglobin, Myoglobin.Hemocyanin,Hemerythrin
Hemoglobin :-
Hemoglobin (American) or haemoglobin (British) abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates (with the exception of the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the rest of the body (i.e. the tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power the functions of the organism in the process called metabolism.
Hemoglobin is involved in the transport of other gases: It carries some of the body's respiratory carbon dioxide (about 20–25% of the total) as carbaminohemoglobin, in which CO2 is bound to the globin protein. The molecule also carries the important regulatory molecule nitric oxide bound to a globin protein thiol group, releasing it at the same time as oxygen.

Hemoglobin (American) or haemoglobin (British) abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates (with the exception of the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the rest of the body (i.e. the tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power the functions of the organism in the process called metabolism.
Hemoglobin is involved in the transport of other gases: It carries some of the body's respiratory carbon dioxide (about 20–25% of the total) as carbaminohemoglobin, in which CO2 is bound to the globin protein. The molecule also carries the important regulatory molecule nitric oxide bound to a globin protein thiol group, releasing it at the same time as oxygen.
Hemoglobin has a quaternary structure characteristic of many multi-subunit globular proteins. Most of the amino acids in hemoglobin form alpha helices, and these helices are connected by short non-helical segments. Hydrogen bonds stabilize the helical sections inside this protein, causing attractions within the molecule, which then causes each polypeptide chain to fold into a specific shape. Hemoglobin's quaternary structure comes from its four subunits in roughly a tetrahedral arrangement.
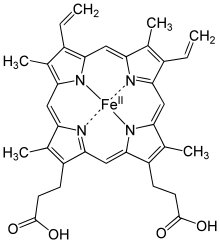
A heme group consists of an iron (Fe) ion (charged atom) held in a heterocyclic ring, known as a porphyrin. This porphyrin ring consists of four pyrrole molecules cyclically linked together (by methine bridges) with the iron ion bound in the center. The iron ion, which is the site of oxygen binding, coordinates with the four nitrogen atoms in the center of the ring, which all lie in one plane. The iron is bound strongly (covalently) to the globular protein via the N atoms of the imidazole ring of F8 histidine residue (also known as the proximal histidine) below the porphyrin ring. A sixth position can reversibly bind oxygen by a coordinate covalent bond,completing the octahedral group of six ligands. Oxygen binds in an "end-on bent" geometry where one oxygen atom binds to Fe and the other protrudes at an angle. When oxygen is not bound, a very weakly bonded water molecule fills the site, forming a distorted octahedron.
Even though carbon dioxide is carried by hemoglobin, it does not compete with oxygen for the iron-binding positions but is bound to the protein chains of the structure.
The iron ion may be either in the Fe2+ or in the Fe3+ state, but ferrihemoglobin (methemoglobin) (Fe3+) cannot bind oxygen.[38] In binding, oxygen temporarily and reversibly oxidizes (Fe2+) to (Fe3+) while oxygen temporarily turns into the superoxide ion, thus iron must exist in the +2 oxidation state to bind oxygen. If superoxide ion associated to Fe3+ is protonated, the hemoglobin iron will remain oxidized and incapable of binding oxygen. In such cases, the enzyme methemoglobin reductase will be able to eventually reactivate methemoglobin by reducing the iron center.
Oxyhemoglobin:-
-The oxygenated form of Hb is called oxyhemoglobin .
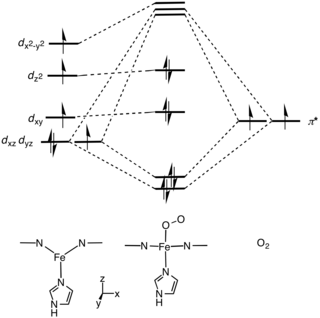
-Fe present in +2 oxidation state,Diamagnetic
-octahedral and low spin complexes (t2g6 eg0)
-Fe-N (porphyrin) Bond short
-The oxyHb adopts relaxed conformation or R-form
Deoxyhemoglobin:-
-The deoxygenated form of Hb(or reduced form of Hb) is called Deoxyhemoglobin
-Fe is present in +2 oxidation state,Paramagnetic
-Octahedral and High spin complex (t2g4 eg2)
-Fe-N Bond large
The deoxyHb held in tensed conformation or T-form
In adult humans, the most common hemoglobin type is a tetramer (which contains four subunit proteins) called hemoglobin A, consisting of two α and two β subunits non-covalently bound, each made of 141 and 146 amino acid residues, respectively. This is denoted as α2β2. The subunits are structurally similar and about the same size. Each subunit has a molecular weight of about 16,000 daltons, for a total molecular weight of the tetramer of about 64,000 daltons (64,458 g/mol). Thus, 1 g/dL = 0.1551 mmol/L. Hemoglobin A is the most intensively studied of the hemoglobin molecules.
In human infants, the hemoglobin molecule is made up of 2 α chains and 2 γ chains. The gamma chains are gradually replaced by β chains as the infant grows.
The four polypeptide chains are bound to each other by salt bridges, hydrogen bonds, and the hydrophobic effect.
Importance of globin protein chain:-
The free heme group(without globin protein chain),the free heme in aq.solution bind with oxygen to form
μ-oxo dimer and that dimer is called Hematin in viz o.s. of Fe = +3 and that is unstable to transport oxygen.
Myoglobin:-
Myoglobin, a protein found in the muscle cells of animals. It functions as an oxygen-storage unit, providing oxygen to the working muscles. Diving mammals such as seals and whales are able to remain submerged for long periods because they have greater amounts of myoglobin in their muscles than other animals do.
There is a close chemical similarity between myoglobin and hemoglobin, the oxygen-binding protein of red blood cells. Both proteins contain a molecular constituent called heme, which enables them to combine reversibly with oxygen. The heme group, which contains iron, imparts a red-brown colour to the proteins. The bond between oxygen and hemoglobin is more complex than that between oxygen and myoglobin and accounts for the dual ability hemoglobin has to transport oxygen as well as to store it.
In contact with venous blood, oxygen combines more readily with myoglobin than it does with hemoglobin, favouring the transfer of oxygen from blood to muscle cells. Thus, the oxygen that the working muscle requires for the energy-producing biochemical reactions is provided.
Myoglobin has been obtained in pure crystalline form from many sources. It has a molecular weightof 16,700, about one-fourth that of hemoglobin. Though the heme portion of all myoglobins is the same, the protein portions vary considerably between species.
Myoglobin belongs to the globin superfamily of proteins, and as with other globins, consists of eight alpha helices connected by loops. Human globin contains 154 amino acids.
Myoglobin contains a porphyrin ring with an iron at its center. A proximal histidine group (His-93) is attached directly to iron, and a distal histidine group (His-64) hovers near the opposite face. The distal imidazole is not bonded to the iron but is available to interact with the substrate O2. This interaction encourages the binding of O2, but not carbon monoxide (CO), which still binds about 240× more strongly than O2.
The binding of O2 causes substantial structural change at the Fe center, which shrinks in radius and moves into the center of N4 pocket. O2-binding induces "spin-pairing": the five-coordinate ferrous deoxy form is high spin and the six coordinate oxy form is low spin and diamagnetic.
Synthetic analogues
Many models of myoglobin have been synthesized as part of a broad interest in transition metal dioxygen complexes. A well known example is the picket fence porphyrin, which consists of a ferrous complex of a sterically bulky derivative of tetraphenylporphyrin. In the presence of an imidazole ligand, this ferrous complex reversibly binds O2. The O2 substrate adopts a bent geometry, occupying the sixth position of the iron center. A key property of this model is the slow formation of the μ-oxo dimer, which is an inactive diferric state. In nature, such deactivation pathways are suppressed by protein matrix that prevents close approach of the Fe-porphyrin assemblies.

Hemocyanin:-
-This is blue coloure compound.Heme group and Cyano group are absent.
-The Cu atom is present at the active site.
-Hemocyanins (also spelled haemocyanins and abbreviated Hc) are proteins that transport oxygen throughout the bodies of some invertebrate animals. These metalloproteins contain two copper atoms that reversibly bind a single oxygen molecule (O2). They are second only to hemoglobin in frequency of use as an oxygen transport molecule. Unlike the hemoglobin in red blood cells found in vertebrates, hemocyanins are not bound to blood cells but are instead suspended directly in the hemolymph. Oxygenation causes a color change between the colorless Cu(I) deoxygenated form and the blue Cu(II) oxygenated form.
structure:-
-At active site- 2Cu atoms are present and each is present in +1 oxidation state.
-It is d10 system-No d-d transition - so deoxyhemocyanin is colourless.
-3 histidine ligands are present on one unit so strained - so tensed (T) shaped geometry due to bulky groups.
-ESR in-active
-When deoxy hemocyanin binds with oxygen gets converted to oxyhemocyanin.
- Dimer form- 2Cu units bonded with 2 bridging oxygens.Cu are present in +2 oxydation state.
-d⁹ system- Diamagnetic due to antiferromagnetic coupling .
-Oxyhemocyanin is color due to ligand to metal charge tranfer (charge transfer between two Cu species.
Spectral properties:-
1.Raman spectroscopy show the symmetric binding of oxygen .This data rules out a mononuclear per oxo complex.
2.The oxyhemocyanin is ESR/EPR in active .This indicate that these strong anti ferromagnetic coupling b/w 2Cu atoms and hence it is diamagnetic in nature.
3.The IR spetroscopy shows that the frequancy is 755 cm viz is less than that indicate oxygen is present in peroxide form in oxy hemocyanin.
Hemerythrin:-
Hemerythrin is an oligomeric protein responsible for oxygen (O2) transport in the marine invertebrate phyla of sipunculids, priapulids, brachiopods, and in a single annelid worm genus, Magelona. Myohemerythrin is a monomeric O2-binding protein found in the muscles of marine invertebrates. Hemerythrin and myohemerythrin are essentially colorless when deoxygenated, but turn a violet-pink in the oxygenated state.
Hemerythrin does not, as the name might suggest, contain a heme. The names of the blood oxygen transporters hemoglobin, hemocyanin, hemerythrin, do not refer to the heme group (only found in globins), instead these names are derived from the Greek word for blood. Recent evidence has revealed hemerythrin to be a multi-functional protein – contributing to innate immunity and anterior tissue regeneration in worms.
O2 binding mechanism
The mechanism of dioxygen binding is unusual. Most O2 carriers operate via formation of dioxygen complexes, but hemerythrin holds the O2as a hydroperoxide (HO2, or -OOH−). The site that binds O2 consists of a pair of iron centres. The iron atoms are bound to the protein through the carboxylate side chains of a glutamate and aspartates as well as through five histidine residues. Hemerythrin and myohemerythrin are often described according to oxidation and ligation states of the iron center:
| Fe2+—OH—Fe2+ | deoxy (reduced) |
| Fe2+—OH—Fe3+ | semi-met |
| Fe3+—O—Fe3+—OOH− | oxy (oxidized) |
| Fe3+—OH—Fe3+— (any other ligand) | met (oxidized) |
The uptake of O2 by hemerythrin is accompanied by two-electron oxidation of the diferrous centre to produce a hydroperoxide (OOH−) complex. The binding of O2 is roughly described in this diagram:
Deoxyhemerythrin contains two high-spin ferrous ions bridged by hydroxyl group (A). One iron is hexacoordinate and another is pentacoordinate. A hydroxyl group serves as a bridging ligand but also functions as a proton donor to the O2 substrate. This proton-transfer result in the formation of a single oxygen atom (μ-oxo) bridge in oxy- and methemerythrin. O2 binds to the pentacoordinate Fe2+ centre at the vacant coordination site (B). Then electrons are transferred from the ferrous ions to generate the binuclear ferric (Fe3+,Fe3+) centre with bound peroxide (C)





Comments
Post a Comment